| This week's sponsor is Bracket. | |  | |
Featured Story Wednesday, February 22, 2017 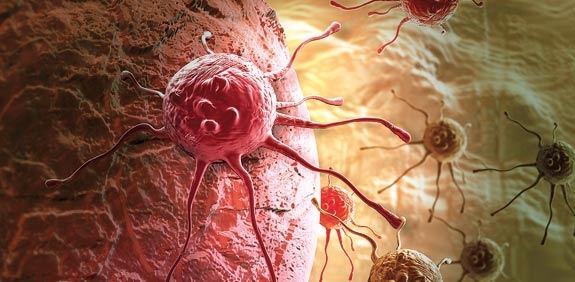 Argos Therapeutics’ hopes of winning approval for its cancer vaccine candidate rocapuldencel-T have taken a major blow. The independent monitoring committee recommended the phase 3 trial is stopped for futility after getting a look at interim results, wiping 65% off Argos' stock and leaving it to scour through the data in search of a path forward for its lead candidate. Argos Therapeutics’ hopes of winning approval for its cancer vaccine candidate rocapuldencel-T have taken a major blow. The independent monitoring committee recommended the phase 3 trial is stopped for futility after getting a look at interim results, wiping 65% off Argos' stock and leaving it to scour through the data in search of a path forward for its lead candidate.
Wednesday, February 22, 2017 New Jersey oncology biotech PMV Pharma has seen a healthy $74 million in funding from its Series B with help from InterWest Partners, Topspin Biotech Fund (which led this round), and its Series A lead investor OrbiMed. Wednesday, February 22, 2017 Apitope has posted phase 2a data on the multiple sclerosis drug Merck KGaA walked away from last year. The single-arm study linked immunotolerizing peptide-based candidate ATX-MS-1467 to a drop in total and new neurological lesions, giving Apitope the confidence to forge ahead with plans to run a phase 2b trial. Tuesday, February 21, 2017 From startups to leading global companies, Greater Fort Lauderdale provides a stimulating and supportive business environment for success in the life sciences. Wednesday, February 22, 2017 People with celiac disease could be freed from the gluten-free aisle of the supermarket if a therapeutic approach developed by ImmusanT proves effective in trials. Tuesday, February 21, 2017 GE Ventures has entered a strategic alliance with the Feinstein Institute, the research arm of New York’s Northwell Health, to advance new bioelectronic therapies and diagnostics for a range of diseases. Wednesday, February 22, 2017 Activist investor Carl Icahn has moved into Bristol-Myers Squibb, touching off more megamerger speculation. After all, the company has seen a major reversal of fortunes since its immunotherapy Opdivo fell short in a key lung cancer study last fall, and just yesterday, it bowed to another activist by adding three directors to its board. | This week's sponsor is RAPS. | |  | |
| | | Ocular Therapeutix says the FDA will look at its NDA resubmission for Dextenza in eye pain after surgery. Release Oncternal has closed an $18.4 million Series B round to help work on its anti-ROR1 blood cancer program, as well as other pipeline projects. Statement RNA biotech Moderna Therapeutics has appointed Israel Ruiz, EVP and treasurer at MIT, as the latest member to its board. Release | |
|
Resources Presented By: Veeva Share your thoughts on the life sciences industry’s progress in reducing system and process complexity to improve study execution. The first 50 qualified respondents will receive a $10 Amazon gift card, as well as a $10 donation made to Doctors Without Borders in their name. Finish the survey today! Sponsored By: Veeva Learn over a dozen best practices for deploying a global content system. Read Whitepaper. Sponsored By: Veeva Gartner provides insightful research on preparing for IDMP compliance. Read Whitepaper. Sponsored By: Veeva Learn How to Create a Unified RIM Environment for IDMP. Find out. Sponsored By: Veeva The largest survey of TMF owners reveals drivers and trends in improving inspection readiness and shortening trial time. Sponsored By: Salesforce Learn how the healthcare and life science industry is using agile application development to drive innovation and accelerate their business with the Salesforce Platform. |