| |
Sept. 25 - 26, 2024 | San Diego, CA Learn out of the box strategies for MSLs to engage with KOLs and HCPs post-pandemic, successful approaches to establishing medical communications plans ahead of a launch. Complimentry Passes Available! Learn More. .png)
|
|
Today’s Big NewsAug 7, 2024 |
|
Transform the Industry with Your Innovation! Submissions are now open for the Fierce Life Sciences Innovation Awards. Click to submit your entry and learn more about the categories and criteria. 
|
|
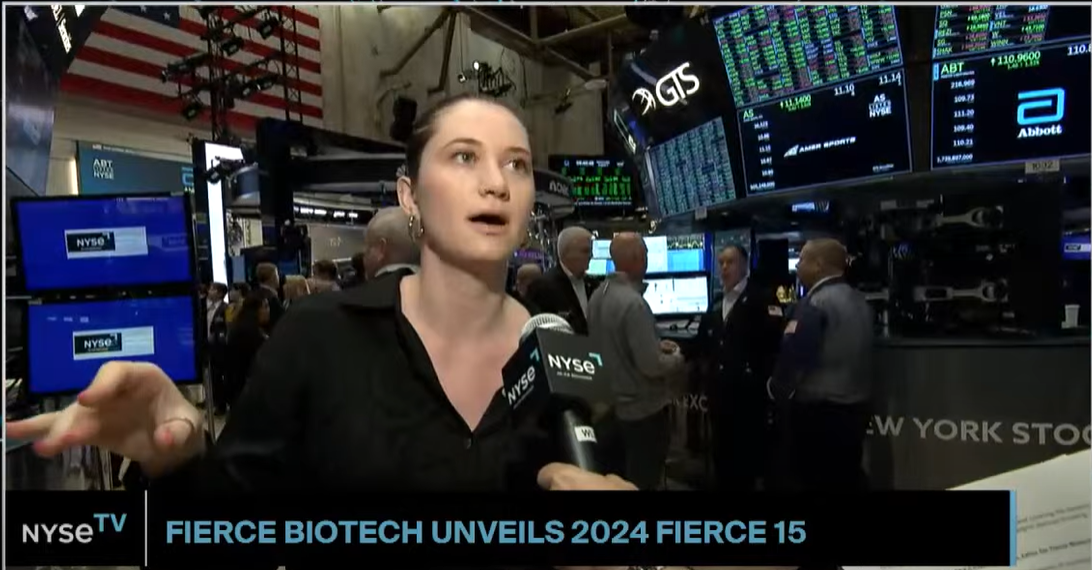
| By Gabrielle Masson Fierce Biotech’s Gabrielle Masson was on the New York Stock Exchange floor this morning to present the 2024 class of Fierce 15 on NYSE TV. Check out Gabrielle’s interview here and the full Fierce 15 list here! |
|
|
|
By Annalee Armstrong Stoke Therapeutics’ Dravet syndrome drug has been freed from a partial hold, clearing the way for the construction of a phase 3 program. |
By James Waldron Exelixis is giving up on its tissue factor-targeting antibody-drug conjugate after concluding the candidate was unlikely to best Pfizer and Genmab’s Tivdak. |
By James Waldron Three weeks after Roche’s Genentech unit walked away from the SHP2 inhibitor pact, Relay Therapeutics has confirmed that it won’t be pushing ahead with a solo project. |
|
Maximize Your Brand Exposure From brand awareness to lead generation, our tailored marketing solutions help you achieve your objectives. Get noticed, start conversations, and drive demand with Fierce Biotech. Learn more now. 
|
|
By Nick Paul Taylor Precigen is throwing everything behind a push to bring a noncancerous tumor gene therapy to market, driving it to lay off 20% of its employees and pause a raft of other programs to stretch its limited funds. |
By Heather Landi Neuralink has implanted its device in a second patient, according to the startup's owner Elon Musk. |
By Gabrielle Masson,Annalee Armstrong,Darren Incorvaia,Max Bayer Welcome to Fierce Biotech's Fundraising Tracker, 2024's version. |
By Darren Incorvaia Full-service contract research organization Rho has named neurologist Andrew Feigin, M.D., its new chief medical officer. Feigin is an expert in movement disorders such as Parkinson’s disease. |
By Angus Liu Servier's Voranigo, or vorasidenib, is now the first systemic therapy for low-grade IDH-mutant brain cancer. |
By Kevin Dunleavy Novo Nordisk’s attempt to gain an approval to treat heart failure patients with its obesity drug Wegovy has been put on hold. In its quarterly earnings presentation, the Danish company said that after discussions with the FDA, it has pulled its filing and expects to resubmit in early 2025. |
By Kevin Dunleavy Amgen’s roller coaster ride known as Tepezza is ascending again. Is the thyroid eye disease treatment finally gaining momentum that is sustainable? In the second quarter, Tepezza generated $479 million in sales for an increase of 8% year over year and 13% sequentially. It was Tepezza’s highest quarterly sales figure since the fourth quarter of 2022. |
By Ben Adams From sounding like a companion of Thor speciality biopharma Aeterna Zentaris is no more: Meet COSCIENS Biopharma. |
Fierce podcasts Don’t miss an episode |
| This week on “Podnosis” we're discussing a new payment model that could affect thousands of hospitals across the country. It's called TEAM, and it stands for Transforming Episode Accountability Model. It's a mandatory bundled payment program that covers five common procedures. |
|
|---|
|
|
|
Thursday, September 5, 2024 | 12pm ET / 9am PT Liquid biopsy is emerging as a revolutionary technology, offering swift and precise diagnostics. However, it still faces key challenges. Join us to learn more about the collaborative efforts of SOPHiA GENETICS, Memorial Sloan Kettering Cancer Center, and AstraZeneca that are aimed at addressing disparities in cancer care. Register now. 
|
|
Whitepaper This paper explores the costs of setting up a supply chain in Europe for a new biopharma therapy. It outlines key cost drivers & the various levers decision makers can use to affect those costs. Sponsored by: AIM |
Whitepaper Download the white paper to learn how this innovative solution can help optimize your mAb production processes. The findings demonstrate the system's robust capability to sustain strong cell growth and high productivity, facilitating a smooth transition from laboratory to production scale. Sponsored by: Thermo Fisher Scientific |
Whitepaper The definitive report on the state of our industry. Readers will gain an understanding of key indicators to monitor, future predictions, and guidance for investors and founders navigating the therapeutic enabling tools and services, healthtech and techbio sectors through 2024 and beyond. Sponsored by: AVANT BIO |
On Helix Date: 4 July 2024 - Location: Babraham Research Campus, Cambridge UK |
|
| |
|

.png)