| Dear Voornaam
Welcome to your weekly Cancer Research UK Research Update email.
Cancer Grand Challenges has opened a global conversation to identify a new set of challenges, inviting scientists, advocates, or anyone affected by cancer to submit their ideas to help shape the next round of funding.
But what makes a perfect Cancer Grand Challenge? Hear from Sheona Scales, Head of Research at Cancer Grand Challenges, about what she hopes to see from this consultation, and access her valuable advice on how to best present a Grand Challenge idea. |
|
|---|
|
|---|
|
|
Funding & Research Opportunities |
|
|---|
|
|---|
| | | Applications accepted all-year round |
|
|---|
|
|---|
| | | Applications accepted all-year round |
|
|---|
|
|---|
|
|
 ALETA-001 RECEIVES INNOVATION PASSPORT DESIGNATION The Medical Health Regulatory Agency granted an Innovation Passport for ALETA-001, Aleta Biotherapeutics CAR-T cell engager candidate. Developed in partnership with our Centre for Drug Development, ALETA-001 will potentially benefit people with B-cell lymphoma and leukaemia who stop responding to CD19 CAR-T cell therapy. Currently, over half of patients treated with CD19 CAR-T cell therapy relapse, mostly due to reduction or loss of CD19 expression.
Receiving a passport accelerates the regulatory process by initiating a dialogue with the agency early in the application process. The MHRAs Innovative Licensing and Access Pathway assists companies with defining regulatory and development features of a novel therapeutic and anticipating any potential regulatory pitfalls that may arise.
We are collaborating with Aleta Biotherapeutics to advance the early phase clinical development of ALETA-001. An initial clinical trial is expected to commence in 2023. |
|
|---|
|  LAUNCHING CANCER RESEARCH HORIZONS ONCOLOGY DEVELOPMENT PROGRAMME Cancer Research Horizons exists to fast-track innovative science into cancer patient benefit and they're growing their entrepreneurial programme initiative by collaborating with Lean Life Science to launch the flagship Oncology Development Programme (ODP2). This insights-driven programme will provide support to the UKs next big innovation, to improve diagnosis and treatment of cancer.
ODP2 will give successful applicants access to bespoke, expert support including scientific review, clinical study design, technical gap analysis, financial and business modelling, and integrated project planning.
If you have a defined oncology-focused innovation, and youre UK-based, find out more on how to apply. |
|
|---|
|
|---|
|
|
POSITIVE PHASE 3 RESULTS POINT TO PROMISE OF CAPIVASERTIB TREATMENT In October, AstraZeneca reported positive results from the CAPItello-291 Phase 3 trial for capivasertib. The clinical trial success moves a much needed, new therapeutic option one-step closer to reaching breast cancer patients who have a recurrence or disease progression following endocrine therapy. Notably, capivasterib is a first-in-class therapeutic originally discovered as part of a collaboration between the Institute of Cancer Research, Cancer Research Horizons and Astex Pharmaceuticals. Broadening the therapeutics options for patients, capivasterib is the first clinical asset to reach Phase 3 from a dedicated discovery platform for developing and commercialising novel small molecule inhibitors of PKB to use as anti-cancer agents. |
|
|---|
|
|---|
|
|
 FOLLOW US ON LINKEDIN Weve started a new research page on LinkedIn for our research community.
Follow us at Cancer Research UK Science and Innovation to stay up to date with research news, opportunities, events and connect with researchers from across the globe. |
|
|---|
|  SHELBY BARNET WINS BIOANALYSIS RISING STAR AWARD Congratulations to the talented Dr Shelby Barnett, who took home the Bioanalysis Zones Rising Star Award in recognition of her work as an early-career scientist specialising in bioanalysis.
Shelby is a Cancer Research UK-funded postdoc working within the Newcastle Cancer Centre Pharmacology Group at Newcastle University, where she runs a therapeutic drug monitoring programme that supports dosing decisions for patients with challenging childhood cancers.
Shelby was also recently awarded the British Pharmacology Bill Bowman prize. |
|
|---|
|
|---|
|
|
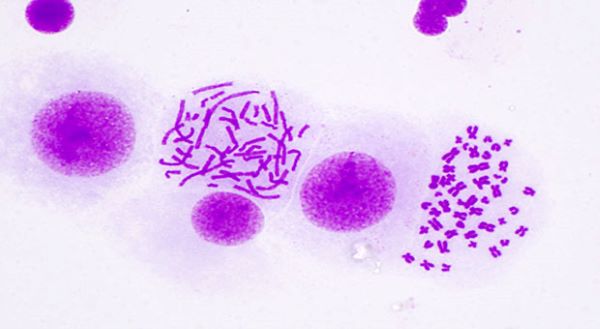 EXPLORING ecDNA AND ITS ROLE IN CANCER DEVELOPMENT What is extrachromosomal DNA, what role does it play in the development of cancer and what has all this got to do with TRACERx, our flagship lung cancer study?
In our latest research feature, Dr Chris Bailey from The Francis Crick Institute tells us how ecDNA challenges traditional models of tumour evolution and drug resistance and why TRACERx has been crucial to push forward our understanding of this molecule. |
|
|---|
|  RESEARCH WITH INTEGRITY In our new column on research integrity, Catherine Winchester, Senior Research Adviser at the CRUK Beatson Institute, talks about the importance of pre-submission manuscript reviews and why researchers should view integrity advisors as a helpful resource rather than the science police.
Catherine believes her role is about raising the standards of scientific communication and encouraging best practice in open, transparent reporting. |
|
|---|
|
|---|
|
|
JOIN OUR BRINGING BIOLOGY TO PREVENTION RESEARCH WEBINAR Join Kay-Tee Khaw, Karen Brown and David Crosby for insights into the future direction for cancer prevention and how understanding the underlying biology of cancer can help answer the big questions about prevention.
Dont miss the chance to ask your burning questions on this new funding scheme for bringing biology to prevention research join us at 2pm GMT on 23 November. |
|
|---|
|
|---|
|
|
| | | Online 2:00 PM 23 November 2022 |
|
|---|
|
|---|
| | | Online 1:00 PM 29 November 2022 |
|
|---|
|
|---|
| | | Online 1:00 PM 29 November 2022 |
|
|---|
|
|---|
| | | East Sussex, UK 04 December 2022 |
|
|---|
|
|---|
|
|
WHAT DID YOU THINK OF THIS EMAIL? We're always looking for ways to improve. Please give us your feedback by clicking below and leaving a comment. |
|
|---|
|
|---|
|
|
| Been forwarded this email?
Subscribe to our newsletter to stay up-to-date. |
|---|
| |
|---|
|
|
|